Homework #6 - due Thursday, 30 October 2003
1. 1. Problem 6 in chapter 4, p.
82 of Ashcroft and Mermin.

2. Problem 1 in chapter 5, p. 93 of Ashcroft and Mermin.
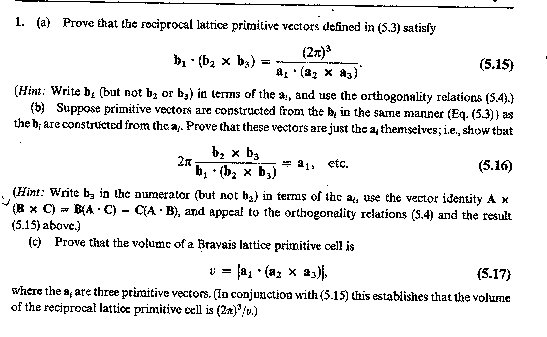
3. AlN crystallizes in the wurzitre lattice with a = 0.31115 nm, and c = 0.49798 nm at 300 K. Determine the number of nitrogen atons per cm in the AlN crystel at 300 K.
4. Problem 1.4 in chapter 1, p. 20 of R. Pierret. (copied below)
5. Up to 910 °C,
iron crystallizes in its a
modification that is BCC. The g
modification that exists above 910 °C
is FCC.Which of the 2 crystal modifications
contains more interstitial space?Which
crystal can accept larger spherical interstitial atoms?Hint:
consider the iron atoms as spheres touching.The
difference in interstitial size allows Carbon atoms to be incorporated
forming high Carbon steel (a range near 1 % C) that is harder than regular
steel.
Homework assignments will appear on the web at: http://www.ece.udel.edu/~kolodzey/courses/eleg621f03.html
Note: On each homework and
report submission, give your name,
the due date, assignment number and the course.